雾霾中硫酸盐的形成机制发表在Atom. Phys. Chem.
Posted 科学之理
tags:
篇首语:本文由小常识网(cha138.com)小编为大家整理,主要介绍了雾霾中硫酸盐的形成机制发表在Atom. Phys. Chem.相关的知识,希望对你有一定的参考价值。
近日,中国科大地学院谢周清教授带领的雾霾关键污染物形成的大气化学机制攻关团队取得重要进展。研究成果发表在国际大气环境权威期刊《Atmospheric Chemistry and Physics》上: Pengzhen He et al., Isotopic constraints on heterogeneous sulfate production in Beijing haze, Atom. Chem. Phys., 18, 5515–5528, 2018。该项研究首次通过17O同位素的非质量分馏(Δ17O)定量揭示了北京APEC前后雾霾硫酸盐的形成机制。
众所周知我国京津冀地区秋冬季面临着严重的雾霾污染,而硫酸盐是雾霾期间PM2.5的主要成分之一,定量了解硫酸盐在雾霾期间的形成机制对于准确预测和防控雾霾非常关键。大气中硫酸盐一般来自于一次直接排放和二氧化硫气体在大气中的气相、液相和非均相反应。由于不同途径的氧化剂不同,形成的硫酸盐中过量17O的数值不同,这种差异与氧同位素的质量无关,被称为非质量分馏(Δ17O)。比如,臭氧(O3)的液相氧化产生的硫酸盐Δ17O高达6.5‰,过氧化氢(H2O2)氧化产生的为0.7‰,氢氧自由基(OH)氧化产生的则为0‰.,基于以上认识,谢周清团队提出了通过分析雾霾事件中硫酸盐17O同位素的变化反演化学反应的路径。
在2014年10月至2015年1月APEC前后,研究团队在北京雁栖湖设置了大气化学观测和悬浮颗粒物采样点。通过分析观测期间的5次雾霾事件,发现PM2.5浓度的12小时平均值最高达到323微克每立方米,硫酸盐的含量最高占到四分之一。化学动力学计算结果表明,不同的雾霾事件,硫酸盐的形成途径不同,比如,2014年10月24日前后发生的雾霾,由于云水含量较高,来自云中液相反应的硫酸盐占了68%,其他4次事件,来自颗粒物表层的非均相反应贡献了42-54%。基于Δ17O约束的计算进一步发现,雾霾期间非均相硫酸盐的产生途径中,过氧化氢(H2O2)的氧化大约贡献了5-13%,臭氧的氧化大约贡献了21-22%,其余则来自于零-Δ17O反应,如四价硫被NO2氧化(S(IV)+NO2)或氧气氧化(S(IV)+O2),平均贡献在66 %至73 %之间。NO2或氧气的氧化依赖于气溶胶pH值即酸碱度,由于稳态和亚稳态状态假设下模型计算得到的气溶胶pH差别很大,因此,S(IV)+NO2和S(IV)+O2反应的相对重要性不同。在稳态假设下, S(IV)+NO2反应是主导的非均相反应,而当高酸性气溶胶存在时,S(IV)+O2反应可能成为主导反应。气溶胶的酸碱度是触发不同非均相化学反应的关键。该研究为定量揭示雾霾硫酸盐生成途径提供了新的方法,为模型预测提供了新的约束条件,并为控制雾霾硫酸盐的形成提供了新的视角。
Table 1. Sulfate isotope assumptions.
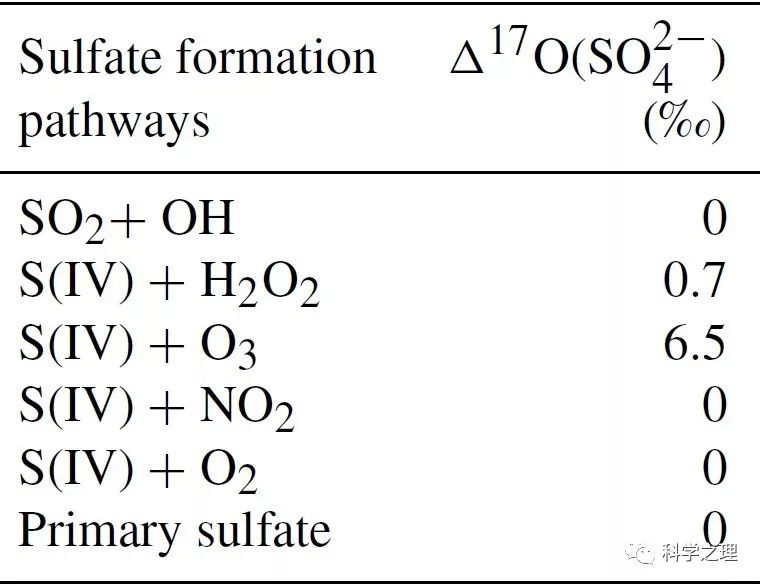
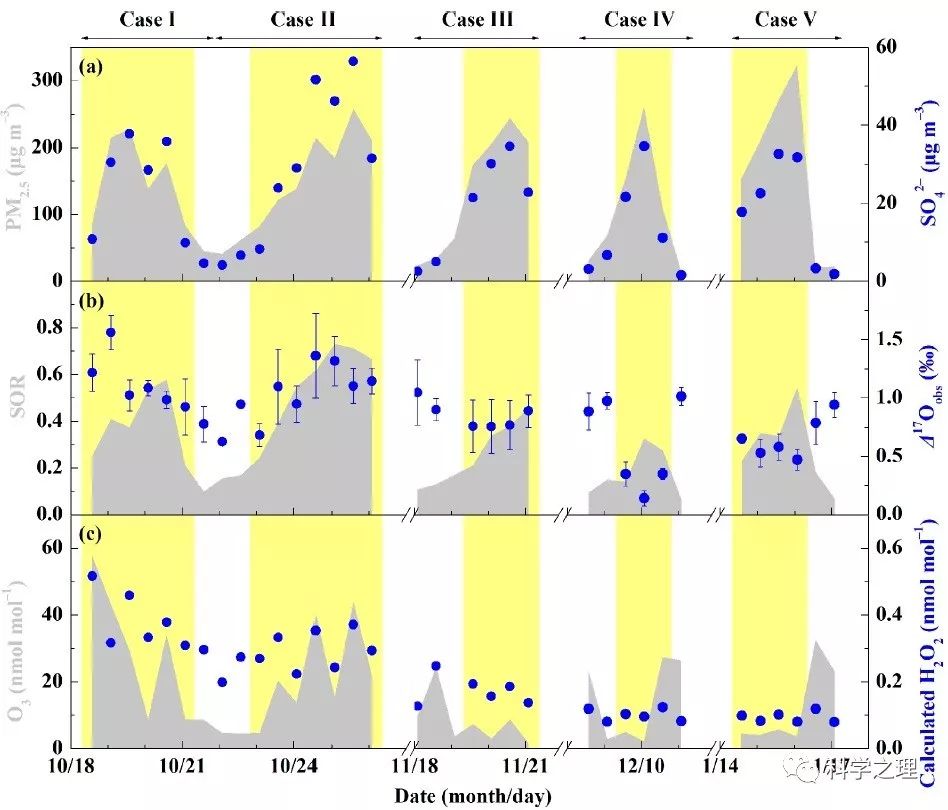
Figure 1. Characteristics of haze events in Beijing (October 2014–January 2015). (a) Temporal evolution of PM2.5 and SO42- concentrations. (b) Temporal evolution of sulfur oxidation ratio (sulfur oxidation ratio (SOR), which equals SO42- molar concentration divided by the sum of SO42- and SO2 molar concentration) and observed Δ17O(SO42-) (Δ17Oobs). (c) Temporal evolution of observed O3 and calculated H2O2. The error bar of Δ17Oobs in (b) is ±1σ of replicate measurements (n=2–4) of each sample. The light yellow shaded area indicates polluted days (PDs, PM2.5≥75 μgm-3). Data used here are 12-hourly averaged values, corresponding with filter samples.

Figure 2. Ternary diagram of possible fractional contribution of different pathways to total sulfate production directly estimated from Δ17Oobs. The colored lines are contour lines of Δ17Oobs, representing possible fractional contribution of sulfate formation via O3( fS(IV)+O3) and H2O2 (fS(IV)+H2O2) oxidation or zero-Δ17O processes (fzero-Δ17O) such as primary sulfate, secondary sulfate formed via OH oxidation, NO2 oxidation, and O2 oxidation. fS(IV)+H2O2 is in the range of 0 to min{Δ17Oobs/0.7‰, (6.5‰-Δ17Oobs)/5.8‰},fS(IV)+O3=(Δ17Oobs-0:7‰×fS(IV)+H2O2/6.5‰, and fzero-Δ17O=(6.5‰-Δ17Oobs-5.8‰×fS(IV)+H2O2/6.5‰.
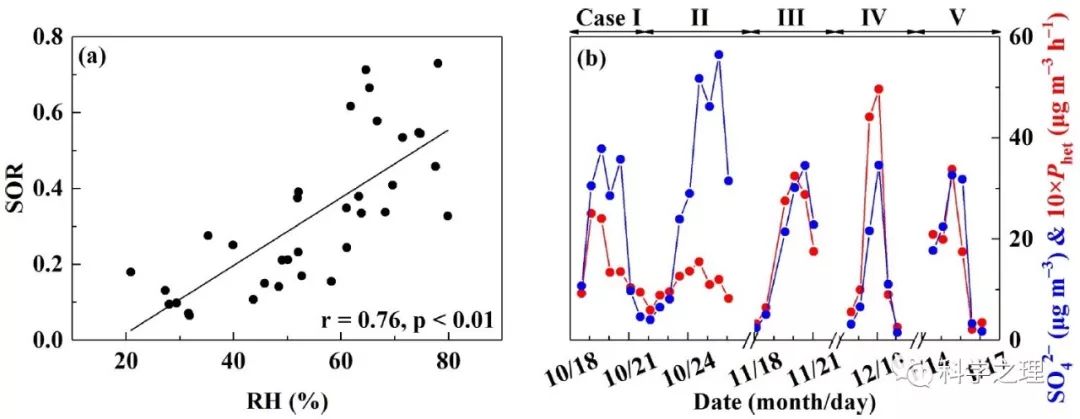
Figure 3. The relationship between relative humidity (RH) and SOR (a) and time series of overall heterogeneous sulfate production (Phet) along with SO42- concentrations (b). The black line in (a) is the linear least-squares fitting line.
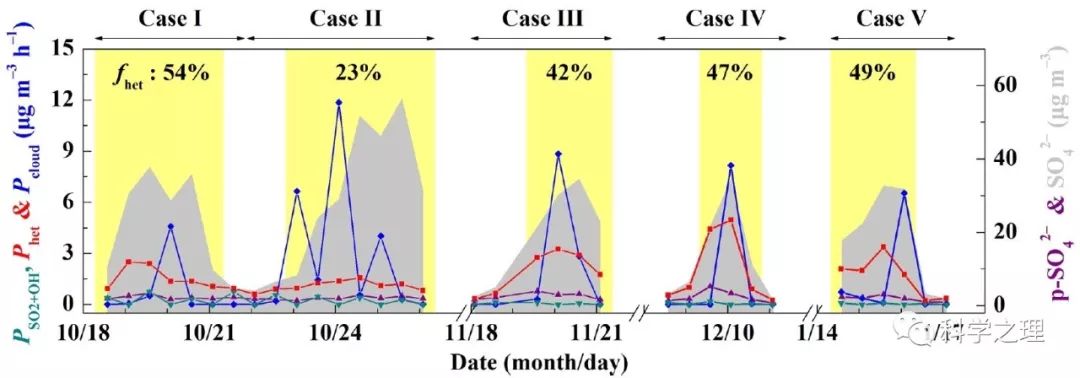
Figure 4. Estimate of different sulfate production pathways. Time series of estimated sulfate production rate via OH oxidation in the gas phase (PSO2+OH), overall heterogeneous reactions on aerosols (Phet) and in-cloud reactions (Pcloud), and concentrations of primary sulfate (p-SO42-) and observed sulfate. fhet represents the fraction of overall heterogeneous sulfate production to total sulfate production during PDs of each case. The light yellow shaded area indicates polluted days (PDs, PM2.5≥75 μg m-3). Data used here are 12-hourly averaged values, corresponding with filter samples.
Table 2. Estimated fractional contribution of different sulfate production pathways during Beijing haze.
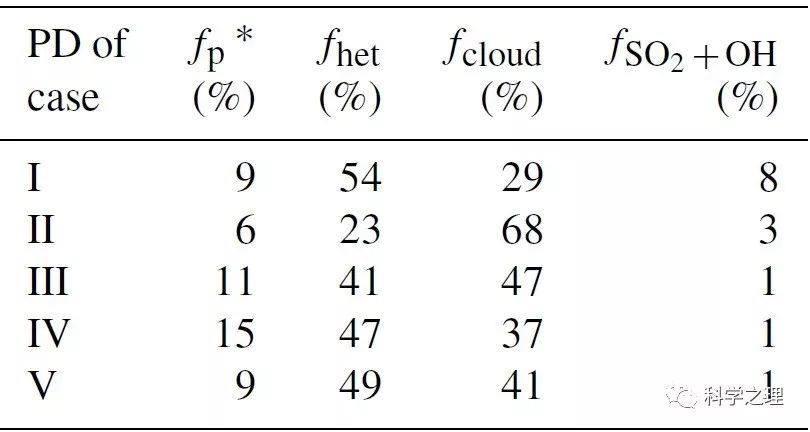
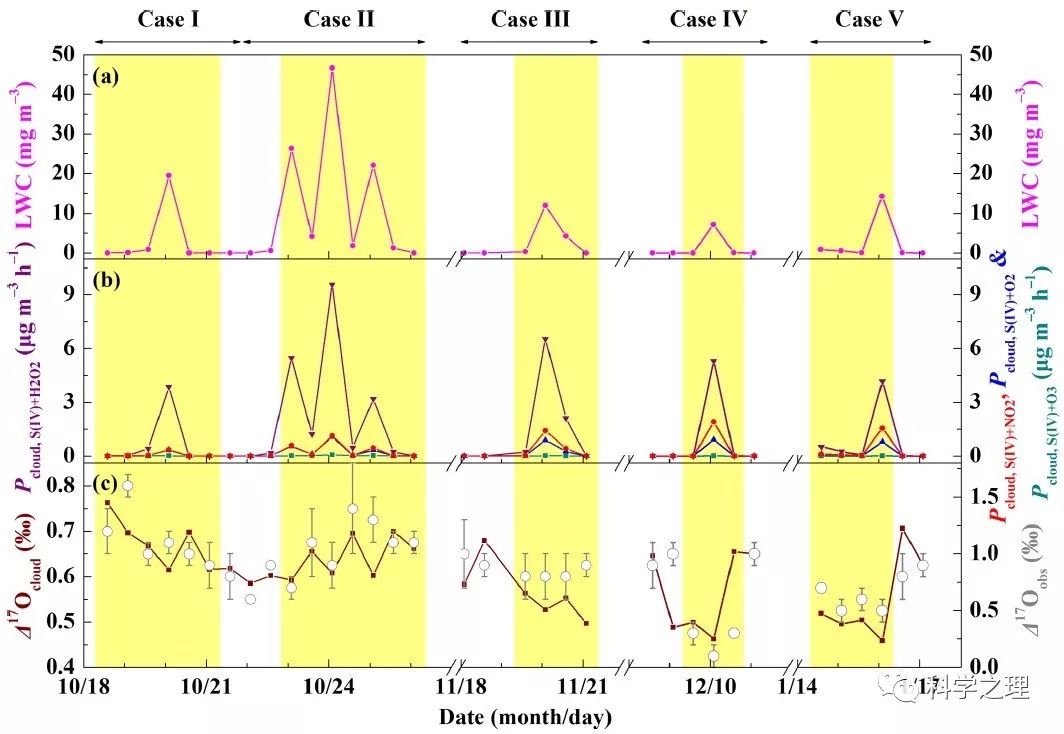
Figure 5. Temporal evolution of cloud liquid water content (LWC, a), in-cloud sulfate production rate via S(IV) oxidation by H2O2, O3, NO2, and O2 initiated by transition metal ions (TMIs) (denoted as Pcloud,S(IV)+H2O2, Pcloud,S(IV)+O3, Pcloud,S(IV)+NO2, and Pcloud,S(IV)+O2, respectively; b) and estimated Δ17O of sulfate produced in clouds (Δ17Ocloud, c). The light yellow shaded area indicates polluted days (PDs, PM2.5≥75 μg m-3). Data used here are 12-hourly averaged values, corresponding with filter samples.
Figure 6. Aerosol parameters during Beijing haze. The aerosol liquid water content (AWC, a), ionic strength (Is, b), and aerosol pH (c) was predicted by ISORROPIA II assuming stable aerosol state and metastable aerosol state. The pH of filtrate was measured using an ion activity meter.
评审专家认为:“为量化北京雾霾期间硫酸盐的非均相反应提供了新的理论框架”,“在应用三氧同位素进行定量识别硫酸盐形成领域向前迈进了一大步”。
论文第一作者为谢周清课题组博士生贺鹏真。
以上是关于雾霾中硫酸盐的形成机制发表在Atom. Phys. Chem.的主要内容,如果未能解决你的问题,请参考以下文章