ASCO2021 | ATM突变mCRPC患者的临床特征与分子标志物的相关性
Posted 泌尿科那点事儿
tags:
篇首语:本文由小常识网(cha138.com)小编为大家整理,主要介绍了ASCO2021 | ATM突变mCRPC患者的临床特征与分子标志物的相关性相关的知识,希望对你有一定的参考价值。
ATM突变mCRPC患者的临床特征与分子标志物的相关性
背景: 共济失调毛细血管扩张突变基因(Ataxia telangiectasia-mutated gene, ATM)是一种丝氨酸/苏氨酸激酶,参与DNA损伤修复(DNA-damage repair, DDR)系统,调节细胞周期检查点、衰老和凋亡。几个临床研究结果证实携带DDR突变的转移性去势抵抗前列腺癌(mCRPC)患者PARP抑制剂(PARPi)治疗有效。然而,PARPi对存在ATM突变的mCRPC患者的疗效有限。我们的目的是探讨携带ATM突变的mCRPC患者的临床特征与分子标志物的相关性。
方法: 三个独立的前列腺癌队列(MCTP,N=61;SU2C-PCF,N=429;MSKCC,N=451)纳入分析。我们选择具有“mCRPC”和“组织学类型:前列腺癌”特征的患者。分析了体系和胚系致病性改变,包括单核苷酸变异(SNVs)、框内插入或缺失(Indels)、移码、纯合缺失和融合。用R软件进行统计分析。
结果: 三个mCRPC队列,产生一个由507名mCRPC患者组成的新队列。中位年龄为62岁(38.6-89岁)。每名患者的活检次数为1-3次。在527例测序标本中,淋巴结组织占39%,骨组织占27%,肝组织占14%。40%的患者曾接受过或正在接受新一代雄激素受体信号抑制剂(ARSI)治疗。共有44名患者(8.7%)存在ATM有害突变,包括10个纯合缺失。共存突变最常见的位置是雄激素受体(AR),其次为TTN/KMT2C/MUC16/TP53。此外,我们还探讨了致病性ATM突变与临床特征的关系。与非ATM突变携带者相比,ATM突变携带者的诊断年龄更小(中位数:59岁 vs. 62.4岁,p=0.022)。在SU2C-PCF队列的亚组分析中,诊断时的前列腺特异性抗原(PSA)和高危患者(GS 8-10)比例在两组间无显著性差异。值得注意的是,我们观察到ATM突变组的肿瘤突变负荷(TMB)显著高于ATM野生组(中位数,2.04 vs. 1.61,p=0.013)。由于ATM突变人群的临床资料有限,因此没有比较总生存(OS)。
结论: 本研究涉及3个队列,确定了致病性体系或胚系ATM突变的患病率,并探讨了ATM突变患者的临床特征和分子标志物的相关性。ATM基因突变的前列腺癌患者在诊断时年龄更小,同时,TMB显著升高意味着ATM突变的mCRPC患者可能从潜在免疫治疗获益。
评论
这是今年ASCO网络会议上发布的一篇来自中国的关注同源重组修复缺陷(HRD)的mCRPC精准诊治的研究。尽管FDA已经批准奥拉帕利和卢卡帕利用于治疗生物标志物阳性的mCRPC患者。但迄今为止,仍然没有可靠的分子标志物用于分子分层。临床研究也发现,不是所有非BRCA突变患者获益于PARPi治疗。研究者在注意到,ATM突变和BRCA突变患者PARPi治疗的疗效差异后,着手探讨携带ATM突变的mCRPC患者的临床特征和分子标志物的相关性。在507例患者中,只有44名患者(8.7%)存在ATM的有害突变,其中纯合缺失仅仅10个。共存突变最常见于雄激素受体(AR),其次是TTN/KMT2C/MUC16/TP53。此外,研究者还发现,与非ATM突变携带者相比,携带者在诊断时年龄更小。而且ATM突变组的TMB显著高于ATM野生组(中位数,2.04 vs. 1.61,p=0.013)。 这一研究结果的重要意义是: 2019年公布的PROFOUNDIII期临床研究发现,在接受奥拉帕利治疗的携带HRD的mCRPC患者中,ATM突变与BRCA1/2突变患者比较,两组人群获得的rPFS(3.6月vs.7.4月,p<0.0001)和OS(15.1月vs.18.5月,p=0.0173)有显著性差异 [1] 。因此,需要探究这种差异的本质原因,为ATM突变mCRPC患者更有效治疗方法提供依据和策略。 研究资料证实,携带BRCA2患者发生前列腺癌的年龄与没有突变者比较提前10年,因此EAU指南和NCCN指南都推荐携带BRCA突变的患者接受前列腺癌筛查的年龄最好是在40岁,而不是在50岁时进行。同时,研究也发现BRCA2突变男性有更高的非局限性疾病的发现率和更坏的预后风险。在接受局部治疗后,BRCA突变者预后更差。既往的研究没有发现ATM突变具有这些临床特征,该研究发现ATM突变前列腺癌人群具有年轻化趋势。
研究者还提到了共存突变(coexistence mutations)现象,并报道ATM突变mCPRC患者中,最常见的共存突变基因雄激素受体(AR)基因,其次是TTN/KMT2C/MUC16/TP53。体外动物模型研究发现,PIK3CA突变和PTEN缺失在前列腺癌中共存,能加速肿瘤的发生,促进CRPC的发生[2]。其他癌种的临床研究发现,共存突变与很多靶向药物的耐药密切相关。在EGFR突变晚期NSCLC的研究中发现,超过92.9%的患者有1种以上的共存突变,大部分(89.8%)为功能性突变,功能性驱动基因突变包括PIK3CA,CTNNB1,BRAF,CDK6,MET,MYC等基因。EGFR与CTNNB1或PIK3CA发生共存突变时会促进转移或EGFR-TKI耐药,并且多基因突变与EGFR- TKI一线治疗患者缓解率和生存负相关[3,4]。前列腺癌领域内PARP抑制剂耐药机制尚不清楚。但研究发现,在初始PARPi的治疗压力下出现逆转BRCA突变,获得性逆转可以恢复BRCA功能,引起对PARPi和/或铂类化疗的耐药性[5]。共存突变可能是影响ATM突变对PARPi响应和耐药的重要参与因素之一。同时,研究还发现,ATM突变前列腺癌患者有比较高的TMB,这也与NCCN指南推荐派姆单抗(Pembrolizumab)用于伴有MSI-H或dMMR表达的mCRPC治疗的意见一致。但有意思的是,在2021年ASCO会议中,另一篇研究(摘要5063)却发现,与BRCA2突变型前列腺癌比较,ATM突变型前列腺癌不太可能共发生P53突变。同时研究者还发现,ATM突变型前列腺癌患者不太可能有诸如dMMR/MSI-H或TMB>=10等这些免疫治疗反应标记物[6]。
这可能与ATM蛋白在同源重组(HR)通路中复杂的功能有关。ATM是通过HR启动DNA双链断裂(DSB)修复所必需。在ATM信号通路中,ATM位于DNA损伤途径的核心位置,其在DNA双链断裂的前提下被激活,并通过多种途径发挥作用,虽然各个通路之间存在大量的串扰,但其下游DNA修复的主要底物是MRN复合体(MRE11、RAD50和NBS1,统称为MRN复合体)、BRCA1、RAD51和P53;参与细胞周期停止的底物是通过P53和检查点激酶起作用的SMC1和CIP/KIP蛋白家族;染色体重塑的底物是CHK1;参与凋亡的底物是c-Abl、P53、Chk2、E2F1、P73和NFkB(图8)[7]。此外,有研究证明ATM不仅用于启动HR,而且用于完成HR[8]。
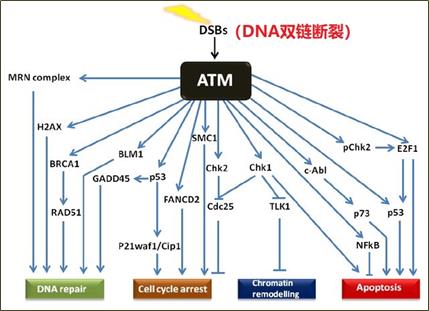
图8 ATM信号通路
Abtract:e17019
Authors:
Guo Xi, Qing WANG, Qin Zhang, Qianqian Duan, Yaqin Liu;
Hunan Provincial People's Hospital, the First Affiliated Hospital of Hunan Normal University, Changsha, China;
Jiangsu simcere diagnostics Co,.Ltd, Guangzhou, China;
The Medical Department, Jiangsu Simcere Diagnostics Co, Ltd, Nanjing Simcere Medical Laboratory Science Co, Ltd,
The State Key Lab of Translational Medicine and Innovative Drug Development, Jiangsu Simcere Diagnostics Co, Ltd, Nanjing, China;
The Medical Department, Jiangsu Simcere Diagnostics Co, Ltd, Nanjing, China
Background:
Ataxia telangiectasia-mutated(ATM)is a serine/threonine kinase involved in DNA-damage repair (DDR) system and regulating cell cycle checkpoints, senescence, and apoptosis. Several clinical studies provide evidence about the DDR-mutated metastatic castration-resistant prostate cancer (mCRPC) benefit from PARP inhibitors (PARPi). However. the efficacy of PARPi was limited for mCRPC patients harboring ATM aberrations. We aimed to determine the association of ATM mutations with clinical and molecular features in patients with mCRPC.
Methods:
A total of three independent prostate cancer cohorts (MCTP, N = 61; SU2C-PCF, N = 429; MSKCC, N = 451) were used for analysis. We selected patients with characteristics “mCRPC” and “histology type: prostate adenocarcinoma”. Both the somatic and germline pathogenic alterations were analyzed, including single nucleotide variations (SNVs), in-frame insertions or deletions (Indels), frameshifts, homozygous deletions and fusions. The statistical analysis was conducted by R software (v.3.6.2).
Results:
We merged the three mCRPC cohorts, generating a new cohort consisting of 507 mCRPC patients. The median age was 62 (range, 38.6-89). The number of biopsies was 1-3 per patient. Of the 527 sequenced specimens, 39% were lymph node, 27% were bone, and 14% were liver. Forty percent of the patients had exposed to or on-treatment with next-generation androgen receptor signaling inhibitor (ARSI). A total of 44 patients (8.7%) harbored deleterious variations in ATM, including 10 homozygous deletions. The coexistence mutations were most commonly located in androgen receptor (AR), followed by TTN/KMT2C/MUC16/TP53. Furthermore, we explored the association of pathogenic ATM alterations with clinical features. Compared with noncarriers of ATM mutations , carriers were diagnosed at a younger age (median: 59 vs 62.4, P = 0.022). In the subgroup from SU2C-PCF cohort, prostate specific antigen (PSA) at diagnosis and the proportion of Gleason score between 8-10 were analyzed, both showed no significant differences between the two groups. Notably, we observed a statistically significant higher TMB in ATM-altered group than ATM wildtype group (median, 2.04 vs 1.61, P = 0.013). Overall survival (OS) was not compared because of the limited clinical outcomes in ATM-mutated population.
Conclusions:
This study involving 3 cohorts identified the prevalence of pathogenic somatic or germline ATM mutations and explored the association of ATM alterations with both clinical and molecular features. Patients with ATM mutations tended to diagnose prostate cancer at an earlier age. Meanwhile, significantly higher TMB might imply potential immunotherapy opportunities in ATM-altered mCRPC without standard-of-care approaches.
向上滑动阅览
【参考文献】
[1] Hussain, M.; Mateo, J.; Fizazi, K., et al. PRPROfound: Phase III study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations. In Proceedings of the ESMO Congress 2019, Barcelona, Spain, 27 September–1 October 2019.
[2]Pearson HB, Li J, Meniel VS, et al. Identification of Pik3ca Mutation as a Genetic Driver of Prostate Cancer That Cooperates with Pten Loss to Accelerate Progression and Castration-Resistant Growth. Cancer Discov. 2018, 8(6):764-779.
[3] Hong S, Gao F, Fu S, et al. Concomitant Genetic Alterations With Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4(5):739-742.
[4] Blakely CM, Watkins TBK, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017, 49(12):1693-1704.
[5] Vidula N, Rich TA, Sartor O, et al. Routine Plasma-Based Genotyping to Comprehensively Detect Germline, Somatic, and Reversion BRCA Mutations among Patients with Advanced Solid Tumors. Clin Cancer Res. 2020, 26(11):2546-2555.
[6] Charles J. Ryan, Julie Elaine McGrath, et al. Association of ATM mutations in metastatic prostate cancer with differential genomic alteration profiles from homologous recombination deficient and proficient tumors. J Clin Oncol 39, 2021 (suppl 15; abstr 5063).
[7] Khalil HS, Tummala H, Chakarov S, et al. Targeting ATM pathway for therapeutic intervention in cancer. Biodiscovery 2012; 1: 3.
[8] Bakr A, Oing C, Köcher S,et al. Involvement of ATM in homologous recombination after end resection and RAD51 nucleofilament formation. Nucleic Acids Res. 2015, 43(6):3154-66.


声明:本内容仅代表嘉宾观点,不代表学习联盟平台立场。本内容仅供医学药学专业人士阅读,不构成实际治疗建议。转载请后台联系授权,侵权必究!
编辑:王靖
审核:王冬
热点视频推荐 以上是关于ASCO2021 | ATM突变mCRPC患者的临床特征与分子标志物的相关性的主要内容,如果未能解决你的问题,请参考以下文章