2017诺贝尔生理学或医学揭晓:Jeffrey C.Michael Rosbash Michael . Young三人分享
Posted 生物制药快迅
tags:
篇首语:本文由小常识网(cha138.com)小编为大家整理,主要介绍了2017诺贝尔生理学或医学揭晓:Jeffrey C.Michael Rosbash Michael . Young三人分享相关的知识,希望对你有一定的参考价值。
编者按
北京时间 2017年10年2日17:30,当地时间11:30 am,为奖励发现控制昼夜节律的分子机制,瑞典斯德哥尔摩卡罗琳医学院授予Jeffrey C. Hall, Michael Rosbash and Michael W. Young三人2017诺贝尔生理学或医学,三人或分享约800万瑞士法郎奖金。
主要贡献
Life on Earth is adapted to the rotation of our planet. For many years we have known that living organisms, including humans, have an internal, biological clock that helps them anticipate and adapt to the regular rhythm of the day. But how does this clock actually work? Jeffrey C. Hall, Michael Rosbash and Michael W. Young were able to peek inside our biological clock and elucidate its inner workings. Their discoveries explain how plants, animals and humans adapt their biological rhythm so that it is synchronized with the Earth's revolutions.
Using fruit flies as a model organism, this year's Nobel laureates isolated a gene that controls the normal daily biological rhythm. They showed that this gene encodes a protein that accumulates in the cell during the night, and is then degraded during the day. Subsequently, they identified additional protein components of this machinery, exposing the mechanism governing the self-sustaining clockwork inside the cell. We now recognize that biological clocks function by the same principles in cells of other multicellular organisms, including humans.
With exquisite precision, our inner clock adapts our physiology to the dramatically different phases of the day. The clock regulates critical functions such as behavior, hormone levels, sleep, body temperature and metabolism. Our wellbeing is affected when there is a temporary mismatch between our external environment and this internal biological clock, for example when we travel across several time zones and experience "jet lag". There are also indications that chronic misalignment between our lifestyle and the rhythm dictated by our inner timekeeper is associated with increased risk for various diseases.
Our inner clock
Most living organisms anticipate and adapt to daily changes in the environment. During the 18th century, the astronomer Jean Jacques d'Ortous de Mairan studied mimosa plants, and found that the leaves opened towards the sun during daytime and closed at dusk. He wondered what would happen if the plant was placed in constant darkness. He found that independent of daily sunlight the leaves continued to follow their normal daily oscillation (Figure 1). Plants seemed to have their own biological clock.
Other researchers found that not only plants, but also animals and humans, have a biological clock that helps to prepare our physiology for the fluctuations of the day. This regular adaptation is referred to as the circadianrhythm, originating from the Latin words circa meaning "around" and diesmeaning "day". But just how our internal circadian biological clock worked remained a mystery.
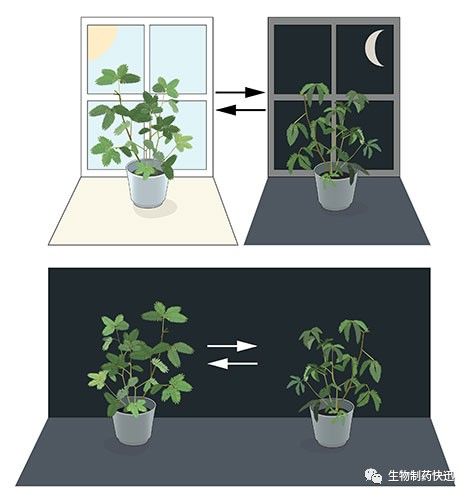
Figure 1. An internal biological clock. The leaves of the mimosa plant open towards the sun during day but close at dusk (upper part). Jean Jacques d'Ortous de Mairan placed the plant in constant darkness (lower part) and found that the leaves continue to follow their normal daily rhythm, even without any fluctuations in daily light.
Identification of a clock gene
During the 1970's, Seymour Benzer and his student Ronald Konopka asked whether it would be possible to identify genes that control the circadian rhythm in fruit flies. They demonstrated that mutations in an unknown gene disrupted the circadian clock of flies. They named this gene period. But how could this gene influence the circadian rhythm?
This year's Nobel Laureates, who were also studying fruit flies, aimed to discover how the clock actually works. In 1984, Jeffrey Hall and Michael Rosbash, working in close collaboration at Brandeis University in Boston, and Michael Young at the Rockefeller University in New York, succeeded in isolating the period gene. Jeffrey Hall and Michael Rosbash then went on to discover that PER, the protein encoded by period, accumulated during the night and was degraded during the day. Thus, PER protein levels oscillate over a 24-hour cycle, in synchrony with the circadian rhythm.
A self-regulating clockwork mechanism
The next key goal was to understand how such circadian oscillations could be generated and sustained. Jeffrey Hall and Michael Rosbash hypothesized that the PER protein blocked the activity of the period gene. They reasoned that by an inhibitory feedback loop, PER protein could prevent its own synthesis and thereby regulate its own level in a continuous, cyclic rhythm (Figure 2A).
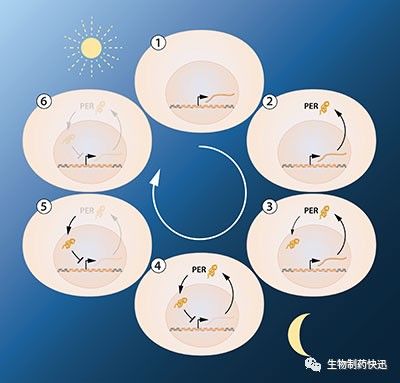
Figure 2A. A simplified illustration of the feedback regulation of the periodgene. The figure shows the sequence of events during a 24h oscillation. When the period gene is active, period mRNA is made. The mRNA is transported to the cell's cytoplasm and serves as template for the production of PER protein. The PER protein accumulates in the cell's nucleus, where the period gene activity is blocked. This gives rise to the inhibitory feedback mechanism that underlies a circadian rhythm.
The model was tantalizing, but a few pieces of the puzzle were missing. To block the activity of the period gene, PER protein, which is produced in the cytoplasm, would have to reach the cell nucleus, where the genetic material is located. Jeffrey Hall and Michael Rosbash had shown that PER protein builds up in the nucleus during night, but how did it get there? In 1994 Michael Young discovered a second clock gene, timeless, encoding the TIM protein that was required for a normal circadian rhythm. In elegant work, he showed that when TIM bound to PER, the two proteins were able to enter the cell nucleus where they blocked period gene activity to close the inhibitory feedback loop (Figure 2B).
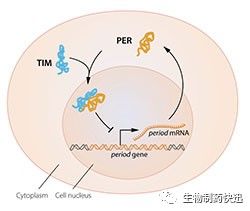
Figure 2B. A simplified illustration of the molecular components of the circadian clock.
Such a regulatory feedback mechanism explained how this oscillation of cellular protein levels emerged, but questions lingered. What controlled the frequency of the oscillations? Michael Young identified yet another gene, doubletime, encoding the DBT protein that delayed the accumulation of the PER protein. This provided insight into how an oscillation is adjusted to more closely match a 24-hour cycle.
The paradigm-shifting discoveries by the laureates established key mechanistic principles for the biological clock. During the following years other molecular components of the clockwork mechanism were elucidated, explaining its stability and function. For example, this year's laureates identified additional proteins required for the activation of the period gene, as well as for the mechanism by which light can synchronize the clock.
Keeping time on our human physiology
The biological clock is involved in many aspects of our complex physiology. We now know that all multicellular organisms, including humans, utilize a similar mechanism to control circadian rhythms. A large proportion of our genes are regulated by the biological clock and, consequently, a carefully calibrated circadian rhythm adapts our physiology to the different phases of the day (Figure 3). Since the seminal discoveries by the three laureates, circadian biology has developed into a vast and highly dynamic research field, with implications for our health and wellbeing.
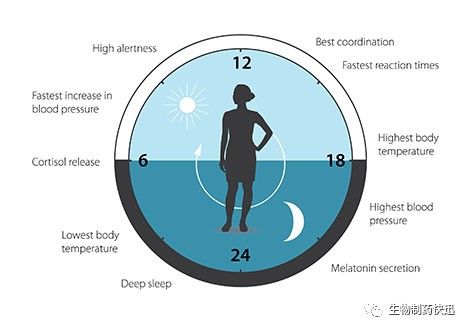
Figure 3. The circadian clock anticipates and adapts our physiology to the different phases of the day. Our biological clock helps to regulate sleep patterns, feeding behavior, hormone release, blood pressure, and body temperature.
历届获奖名单
下面,我们来一起重温一下近十年内的诺贝尔生理学或医学奖获奖科学家的风采~
2006年,安德鲁·法尔(AndrewZachary Fire)和克雷格·梅洛(Craig Cameron Mello)

2006年,美国人安德鲁·法尔和克雷格·梅洛凭借"RNA干扰机制--双链RNA沉默基因" 的发现脱颖而出,成为该年度诺贝尔生理学或医学奖得主。虽奖项名目既涉及生理学,也涉及医学,但针对这两位获奖者及其成果,欧美媒体无不把今年这一奖项称为诺贝尔医学奖。法尔和梅洛以遗传学为切入点,选择针对核糖核酸(RNA)的干扰机制作为研究课题。该机制已被广泛用作研究基因功能的一种手段,并有望在未来帮助科学家开发出治疗疾病的新疗法。
2007年,奥利弗·史密斯(OliverSmithies)和马丁·埃文斯(Martin J. Evans)、马里奥·卡佩基(Mario R. Capecchi)

2007年,来自英美两国的奥利弗o史密斯(OliverSmithies)和马丁o埃文斯(Martin J. Evans)、马里奥o卡佩基(Mario R. Capecchi)三位科学家,获得该年诺贝尔生理学或医学奖。诺贝尔奖评审委员会发布的公报说,三位科学家"在涉及胚胎干细胞和哺乳动物DNA重组方面有着一系列突破性发现",使深入研究单个基因在动物体内的功能并提供相关药物试验的动物模型成为可能,为"基因靶向"技术的发展奠定了基础。
2008年,弗朗索瓦丝·巴尔-西诺西(Fransoise Barré-Sinoussi)和吕克·蒙塔尼(LucMontagnier)、哈拉尔德·楚尔·豪森(Harald zur Hausen)

2008年,三名欧洲科学家因为发现两种引发人类致命疾病的病毒而荣获2008年诺贝尔生理学或医学奖。两名法国科学家弗朗索瓦丝·巴尔-西诺西和吕克·蒙塔尼上世纪80年代发现人类免疫缺陷病毒,使医学界找到了确诊艾滋病病毒感染的办法,延缓了这种病毒的传播。德国科学家哈拉尔德·楚尔·豪森在上世纪70年代开始的研究中发现导致宫颈癌的HPV(人乳头状瘤病毒),为宫颈癌疫苗今天的诞生奠定了基础。
2009年,卡罗尔·格雷德(CarolW.Greider)、伊丽莎白·布莱克本(ElizabethH.Blackburn)、杰克·绍斯塔克(JackW.Szostak)

2009年,美国加利福尼亚旧金山大学的伊丽莎白·布莱克本(ElizabethH.Blackburn)、美国巴尔的摩约翰·霍普金斯医学院的卡罗尔·格雷德(CarolW.Greider)、美国哈佛医学院的杰克·绍斯塔克(JackW.Szostak)因发现端粒和端粒酶保护染色体的机理而获此殊荣。该研究提高了人们对于细胞的理解的深度,对癌症和衰老研究具有重要的意义,有助于未来新治疗方法的发展。
2010年,罗伯特·爱德华(Robert G.Edwards)

2010年,英国生理学家,被称为"试管婴儿之父"的罗伯特·爱德华兹因为在"在试管受精技术方面的发展"获得2010年诺贝尔生理学或医学奖。爱德华兹创立的体外受精技术解决了一个重要的医学难题,即通过体外受精治疗多种不育症。他的贡献使治疗不育症成为可能,包括全球超过10%的夫妇在内的人类因此获益匪浅。
2011年,拉尔夫·斯坦曼因(Ralph M.Steinman)和朱尔斯·霍夫曼(Jules A. Hoffmann)、布鲁斯·博伊特勒(Bruce A. Beutler)

2011年,加拿大科学家拉尔夫·斯坦曼、法国科学家朱尔斯·霍夫曼、美国科学家布鲁斯·博伊特勒因在免疫学领域取得杰出成就而获得2011年诺贝尔生理学或医学奖。其中一半的奖金归于拉尔夫·斯坦曼,获奖理由是"发现树枝状细胞及其在获得性免疫中的作用" ;另一半奖金归于朱尔斯·霍夫曼和布鲁斯·博伊特勒,获奖理由是"先天免疫激活方面的发现"。这三位诺奖得主发现了免疫系统激活的关键原理,从而彻底革新了我们对免疫系统的认识。
2012年,约翰o格登(JohnGurdon) 、山中伸弥(Shinya Yamanaka)

2012年,英国科学家约翰o格登(JohnGurdon) 与日本科学家山中伸弥(Shinya Yamanaka)因在细胞核重新编程研究领域的杰出贡献,获得2012年诺贝尔生理学或医学奖。诺贝尔奖认为,这两位科学家发现了:成熟、分化的细胞可以被重新编程,变成未成熟的细胞,能够发育成机体内所有种类的组织。他们的发现革新了我们对于细胞和有机体如何发育的理解。
2013年,詹姆斯·罗斯曼(James E.Rothman),兰迪·谢克曼(Randy W. Schekman)和托马斯·聚德霍夫(Thomas C. Südhof)

2013年,耶鲁大学细胞生物学系系主任、生物医学教授詹姆斯·罗斯曼(James E. Rothman),加州大学伯克利分校的细胞生物学家兰迪·谢克曼(Randy W. Schekman)和德国生物化学家托马斯·聚德霍夫(Thomas C. Südhof),因"发现细胞内的主要运输系统--囊泡运输的调节机制"获得2013年诺贝尔生理学或医学奖。诺贝尔奖评选委员会说,三位获奖者的研究成果揭示了细胞如何在准确的时间将其内部物质传输至准确的位置,揭示出细胞生理学的一个基本过程。
2014年,爱德华·莫泽(Edvard I.Moser))和梅-布里特·莫泽(May-Britt Moser)、约翰·奥基夫(John O'Keefe)

2014年,来自挪威的科学家爱德华·莫泽(EdvardI. Moser))和梅-布里特·莫泽(May-Britt Moser),以及英国伦敦大学学院教授约翰·奥基夫(John O'Keefe)夫妇共三位科学家,因发现组成大脑定位系统(GPS)的特殊细胞的研究成果获得该年诺贝尔生理学或医学奖。解决了哲学家和科学家几个世纪之久的问题--人类大脑究竟是如何构建一个所处空间的地图,以及在一个复杂的环境中人类大脑如何导航并寻找路径。
2015年,威廉·C·坎贝尔(WilliamC. Campbell)、屠呦呦和大村智(Satoshi ōmura)

2015年诺贝尔生理学或医学奖揭晓,我国科学家屠呦呦获奖!获奖理由是"有关疟疾新疗法的发现"。另外两名获奖科学家为爱尔兰的William C. Campbell和日本的Satoshi ōmura,获奖理由是"有关蛔虫寄生虫感染新疗法的发现"。这两项发现为人类提供了强有力的新武器,从而使得人类能够与这些每年感染亿万人的疾病作斗争。其为人类改善健康、减轻病痛所带来的功德不可估量。
2016年,Yoshinori Ohsumi(大隅良典)
Yoshinori Ohsumi生于1945年,1974年在东京大学获得博士学位!然后进入Rockefeller 大学三年,1988年返回东京大学建立自己的研究室。2009年至今任东京技术研究所教授。
Yoshinori Ohsumi(大隅良典)教授克隆了第一个酵母自噬基因Atg1和LC3,发挥了决定性作用!大隅良典教授是细胞自噬研究的先驱,曾获得京都奖、盖尔德纳国际奖及日本人第2座威利奖(2015年加拿大盖尔德纳奖揭晓)。其专长生物学,特别是分子生物学领域。最知名的成就,是阐明细胞自噬的分子机制和生理功能。由于相关论文被大量引用,因此名列2014年度汤森路透引文桂冠奖。
以上是关于2017诺贝尔生理学或医学揭晓:Jeffrey C.Michael Rosbash Michael . Young三人分享的主要内容,如果未能解决你的问题,请参考以下文章