氮,氧,二氧化碳,天然气,氦,氖,氯 ,一氧化碳,二氧化硫的用处,出自那里,特性
Posted
tags:
篇首语:本文由小常识网(cha138.com)小编为大家整理,主要介绍了氮,氧,二氧化碳,天然气,氦,氖,氯 ,一氧化碳,二氧化硫的用处,出自那里,特性相关的知识,希望对你有一定的参考价值。
最好是英文的,因为我上国际学校,科学老师要我们找,我在谷歌上找了半个小时也没找到。那位大侠知道的就请帮帮我吧!
http://environmentalchemistry.com/yogi/periodic/N.html"CO2" redirects here. For the postal district, see CO postcode area.
Carbon dioxide
IUPAC name Carbon dioxide
Other names Carbonic acid gas; carbonic anhydride; dry ice (solid)
Identifiers
CAS number 124-38-9
PubChem 280
EINECS number 204-696-9
UN number 1013
Solid (dry ice): 1845
Mixtures with Ethylene oxide: 1952,3300
RTECS number FF6400000
SMILES [show]
C(=O)=O
InChI [show]
1/CO2/c2-1-3
ChemSpider ID 274
Properties
Molecular formula CO2
Molar mass 44.0095(14) g/mol
Appearance colorless gas
Density 1,600 g/L, solid; 771 g/L, liquid; 1.98 g/L, gas
Melting point −56.6 °C (216.6 K) −69.9 °F (at 5.185 bar)
Boiling point −78.5 °C (194.7 K) −109.3 °F (sublimes)
Solubility in water 1.45 g/L at 25°C, 100kPa
Acidity (pKa) 6.35 and 10.33
Viscosity 0.07 cP at −78 °C
Dipole moment zero
Structure
Molecular shape linear
Related compounds
Related oxides carbon monoxide; carbon suboxide; dicarbon monoxide; carbon trioxide
Supplementary data page
Structure and
properties n, εr, etc.
Thermodynamic
data Phase behaviour
Solid, liquid, gas
Spectral data UV, IR, NMR, MS
Except where noted otherwise, data are given for
materials in their standard state
(at 25 °C, 100 kPa)
Infobox references
Carbon dioxide (chemical formula: CO2) is a chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom. It is a gas at standard temperature and pressure. Carbon dioxide exists in Earth's atmosphere currently at a globally averaged concentration of approximately 385 parts per million by volume.[1] Carbon dioxide is a greenhouse gas as it transmits visible light but absorbs strongly in the infrared and near-infrared.
Carbon dioxide is used by plants during photosynthesis to make sugars which may either be consumed again in respiration or used as the raw material to produce polysaccharides such as starch and cellulose, proteins and the wide variety of other organic compounds required for plant growth and development. It is produced during respiration by plants, and by all animals, fungi and microorganisms that depend on living and decaying plants for food, either directly or indirectly. It is, therefore, a major component of the carbon cycle. Carbon dioxide is generated as a by-product of the combustion of fossil fuels or the burning of vegetable matter, among other chemical processes. Over very long time scales (thousands to millions of years), concentrations are influenced by emissions from volcanoes and other geothermal processes such as hot springs and geysers and by the dissolution of carbonates in crustal rocks.
Carbon dioxide has no liquid state at pressures below 5.1 atm. At 1 atm it is a solid at temperatures below −78 °C. In its solid state, carbon dioxide is commonly called dry ice.
CO2 is an acidic oxide: an aqueous solution turns litmus from blue to pink.
CO2 in concentrations of 7% to 10% cause dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour
For more details on this topic, see Carbon dioxide (data page).
Carbon dioxide is a colourless, odorless gas. When inhaled at concentrations much higher than usual atmospheric levels, it can produce a sour taste in the mouth and a stinging sensation in the nose and throat. These effects result from the gas dissolving in the mucous membranes and saliva, forming a weak solution of carbonic acid. This sensation can also occur during an attempt to stifle a burp after drinking a carbonated beverage. Amounts above 5,000 ppm are considered very unhealthy, and those above about 50,000 ppm (equal to 5% by volume) are considered dangerous to animal life.[3]
At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m3, about 1.5 times that of air. The carbon dioxide molecule (O=C=O) contains two double bonds and has a linear shape. It has no electrical dipole, and as it is fully oxidized, it is moderately reactive and is non-flammable, but will support the combustion of metals such as magnesium.
Small pellets of dry ice subliming in air.
Crystal structure of dry ice
At −78.51 °C or −109.3 °F, carbon dioxide changes directly from a solid phase to a gaseous phase through sublimation, or from gaseous to solid through deposition. Solid carbon dioxide is normally called "dry ice", a generic trademark. It was first observed in 1825 by the French chemist Charles Thilorier. Dry ice is commonly used as a cooling agent, and it is relatively inexpensive. A convenient property for this purpose is that solid carbon dioxide sublimes directly into the gas phase leaving no liquid. It can often be found in grocery stores and laboratories, and it is also used in the shipping industry. The largest non-cooling use for dry ice is blast cleaning.
Liquid carbon dioxide forms only at pressures above 5.1 atm; the triple point of carbon dioxide is about 518 kPa at −56.6 °C (See phase diagram, above). The critical point is 7.38 MPa at 31.1 °C.[4]
An alternative form of solid carbon dioxide, an amorphous glass-like form, is possible, although not at atmospheric pressure.[5] This form of glass, called carbonia, was produced by supercooling heated CO2 at extreme pressure (40–48 GPa or about 400,000 atmospheres) in a diamond anvil. This discovery confirmed the theory that carbon dioxide could exist in a glass state similar to other members of its elemental family, like silicon (silica glass) and germanium. Unlike silica and germania glasses, however, carbonia glass is not stable at normal pressures and reverts back to gas when pressure is released.
See also: Supercritical carbon dioxide and dry ice
[edit] History of human understanding
Carbon dioxide was one of the first gases to be described as a substance distinct from air . In the seventeenth century, the Flemish chemist Jan Baptist van Helmont observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a "gas" or "wild spirit" (spiritus sylvestre).
The properties of carbon dioxide were studied more thoroughly in the 1750s by the Scottish physician Joseph Black. He found that limestone (calcium carbonate) could be heated or treated with acids to yield a gas he called "fixed air." He observed that the fixed air was denser than air and did not support either flame or animal life. Black also found that when bubbled through an aqueous solution of lime (calcium hydroxide), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, English chemist Joseph Priestley published a paper entitled Impregnating Water with Fixed Air in which he described a process of dripping sulfuric acid (or oil of vitriol as Priestley knew it) on chalk in order to produce carbon dioxide, and forcing the gas to dissolve by agitating a bowl of water in contact with the gas.[6]
Carbon dioxide was first liquefied (at elevated pressures) in 1823 by Humphry Davy and Michael Faraday.[7] The earliest description of solid carbon dioxide was given by Charles Thilorier, who in 1834 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a "snow" of solid CO2.[8]
[edit] Isolation and production
Carbon dioxide may be obtained from air distillation. However, this yields only very small quantities of CO2. A large variety of chemical reactions yield carbon dioxide, such as the reaction between most acids and most metal carbonates. For example, the reaction between hydrochloric acid and calcium carbonate (limestone or chalk) is depicted below:
2 HCl + CaCO3 → CaCl2 + H2CO3
The H2CO3 then decomposes to water and CO2. Such reactions are accompanied by foaming or bubbling, or both. In industry such reactions are widespread because they can be used to neutralize waste acid streams.
The production of quicklime (CaO) a chemical that has widespread use, from limestone by heating at about 850 °C also produces CO2:
CaCO3 → CaO + CO2
The combustion of all carbon containing fuels, such as methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), but also of coal and wood, will yield carbon dioxide and, in most cases, water. As an example the chemical reaction between methane and oxygen is given below.
CH4 + 2 O2 → CO2 + 2 H2O
Iron is reduced from its own oxides with coke in a blast furnace, producing pig iron and carbon dioxide:
2 Fe2O3 + 3 C → 4 Fe + 3 CO2
Yeast metabolizes sugar to produce carbon dioxide and ethanol, also known as alcohol, in the production of wines, beers and other spirits, but also in the production of bioethanol:
C6H12O6 → 2 CO2 + 2 C2H5OH
All aerobic organisms produce CO2 when they oxidize carbohydrates, fatty acids, and proteins in the mitochondria of cells. The large number of reactions involved are exceedingly complex and not described easily. Refer to (cellular respiration, anaerobic respiration and photosynthesis). Photoautotrophs (i.e. plants, cyanobacteria) use another modus operandi: Plants absorb CO2 from the air, and, together with water, react it to form carbohydrates:
nCO2 + nH2O → (CH2O)n + nO2
Carbon dioxide is soluble in water, in which it spontaneously interconverts between CO2 and H2CO3 (carbonic acid). The relative concentrations of CO2, H2CO3, and the deprotonated forms HCO3− (bicarbonate) and CO32−(carbonate) depend on the pH. In neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater, while in very alkaline water (pH > 10.4) the predominant (>50%) form is carbonate. The bicarbonate and carbonate forms are very soluble, such that air-equilibrated ocean water (mildly alkaline with typical pH = 8.2 – 8.5) contains about 120 mg of bicarbonate per liter.
[edit] Uses
Carbon dioxide bubbles in a soft drink.Carbon dioxide is used by the food industry, the oil industry, and the chemical industry.[9] It is used in many consumer products that require pressurized gas because it is inexpensive and nonflammable, and because it undergoes a phase transition from gas to liquid at room temperature at an attainable pressure of approximately 60 bar (870 psi, 59 atm), allowing far more carbon dioxide to fit in a given container than otherwise would. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminum capsules are also sold as supplies of compressed gas for airguns, paintball markers, for inflating bicycle tires, and for making seltzer. Rapid vaporization of liquid carbon dioxide is used for blasting in coal mines. High concentrations of carbon dioxide can also be used to kill pests, such as the Common Clothes Moth.
[edit] Drinks
Carbon dioxide is used to produce carbonated soft drinks and soda water. Traditionally, the carbonation in beer and sparkling wine comes about through natural fermentation, but some manufacturers carbonate these drinks artificially.
[edit] Foods
A candy called Pop Rocks is pressurized with carbon dioxide gas at about 40 bar (600 psi). When placed in the mouth, it dissolves (just like other hard candy) and releases the gas bubbles with an audible pop.
Leavening agents produce carbon dioxide to cause dough to rise. Baker's yeast produces carbon dioxide by fermentation of sugars within the dough, while chemical leaveners such as baking powder and baking soda release carbon dioxide when heated or if exposed to acids.
A carbon dioxide laser.
[edit] Pneumatic systems
Carbon dioxide is the most commonly used compressed gas for pneumatic systems in portable pressure tools and combat robots.
[edit] Fire extinguisher
Carbon dioxide extinguishes flames, and some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide has also been widely used as an extinguishing agent in fixed fire protection systems for total flooding of a protected space, (National Fire Protection Association Code 12). International Maritime Organisation standards also recognise carbon dioxide systems for fire protection of ship holds and engine rooms. Carbon dioxide based fire protection systems have been linked to several deaths. A review of CO2 systems (Carbon Dioxide as a Fire Suppressant: Examining the Risks, US EPA) identified 51 incidents between 1975 and the date of the report, causing 72 deaths and 145 injuries.
[edit] Welding
Carbon dioxide also finds use as an atmosphere for welding, although in the welding arc, it reacts to oxidize most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are brittler than those made in more inert atmospheres, and that such weld joints deteriorate over time because of the formation of carbonic acid. It is used as a welding gas primarily because it is much less expensive than more inert gases such as argon or helium.
[edit] Caffeine removal
Liquid carbon dioxide is a good solvent for many lipophilic organic compounds, and is used to remove caffeine from coffee. First, the green coffee beans are soaked in water. The beans are placed in the top of a column seventy feet (21 m) high. Then super-pressurized carbon dioxide in fluid form at about 93 degrees Celsius enters at the bottom of the column. The caffeine diffuses out of the beans and into the carbon dioxide.
[edit] Pharmaceutical and other chemical processing
Carbon dioxide has begun to attract attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It's used by some dry cleaners for this reason. (See green chemistry.)
In the chemical industry, carbon dioxide is used for the production of urea, carbonates and bicarbonates, and sodium salicylate.
[edit] Biological applications
Plants require carbon dioxide to conduct photosynthesis, and greenhouses may enrich their atmospheres with additional CO2 to boost plant growth, since its low present-day atmosphere concentration is just above the "suffocation" level for green plants. A photosynthesis-related drop in carbon dioxide concentration in a greenhouse compartment can kill green plants. At high concentrations, carbon dioxide is toxic to animal life, so raising the concentration to 10,000 ppm (1%) for several hours can eliminate pests such as whiteflies and spider mites in a greenhouse.
It has been proposed that carbon dioxide from power generation be bubbled into ponds to grow algae that could then be converted into biodiesel fuel.[10] Carbon dioxide is already increasingly used in greenhouses as the main carbon source for Spirulina algae. In medicine, up to 5% carbon dioxide is added to pure oxygen for stimulation of breathing after apnea and to stabilize the O2/CO2 balance in blood. 参考技术A 在百度《百科》分别输入氮,氧,二氧化碳,天然气,氦,氖,氯 ,一氧化碳,二氧化硫就可找到。英文再翻译。
国际视野|Sentinel-5P卫星与全球甲烷追踪器
点击图片上方蓝色字体“慧天地”即可订阅
 转载本文需经【慧天地】许可。
转载本文需经【慧天地】许可。


(点击图片即可查看详细信息)


在了解全球甲烷排放水平方面,最新和最有希望的进展之一就是卫星的使用。如今,运行中的各种不同的卫星在帮助人们估算不同地理区域的甲烷浓度。卫星的主要优势在于,它们可以帮助人们迅速定位大型污染源。一旦发现泄漏事故或者甲烷含量异常的区域,通常可以相对迅速地识别并传达数据信息给当地的相关工作人员。以前,泄漏检测主要依靠使用手持式热像仪来识别排放源,而这种方法既缓慢又麻烦。卫星和其他空中测量方法(例如无人机或飞机)可以提供更快,更全面的视野。卫星还可以帮助我们更好地了解石油和天然气设施产生的甲烷的性质。甲烷浓度变化的数据可用于估计引起这种变化的排放源的排放速率。
哥白尼计划中的Sentinel-5P卫星的目标是提供空间观测数据,以支持空气质量的监测。它将提供臭氧、二氧化氮、甲烷、一氧化碳、甲醛、二氧化硫等空气污染物的测量数据。该卫星携带最先进的Tropomi仪器(对流层监测仪器),结合了SCIAMACHY,OMI和最先进的技术的优势,能够比以往任何时候都更准确地进行污染物的成像。

图1:Sentinel-5P
(图片来源:ESA)
国际能源署于去年启动了“甲烷追踪器(Methane Tracker)”项目,这个交互式的在线工具专注于石油和天然气所产生的排放,这一领域也是能够减少甲烷排放量最多且最具成本效益的领域。目前,Methane Tracker 已在2020年版中进行了全面更新和扩展,用户可以全面了解70多个国家/地区的排放量以及可以降低排放量的技术和措施。除了国际能源署的甲烷追踪项目之外,全球还在采用其他各种举措,以提高世界对石油和天然气行业甲烷排放量和性质的了解。甲烷跟踪器更新不仅包括国际能源署的最新估算,而且还首次包括了其他来源的可比数据。
全球领先的数据分析公司Kayrros使用欧空局哥白尼计划Sentinel-5P卫星的数据并结合机器学习方法,建立了一个探测和量化模型,追踪甲烷排放的源头,并对世界各地的能源和自然资源进行重点监测。除此之外,他们的甲烷追踪器还利用将于2020-2022年投入使用的新卫星,增加平台的覆盖范围和灵敏度。甲烷追踪器还可以整合来自地面和无人机传感器的专有数据源。这些技术的进展意味着甲烷含量现在可以在全球范围内的资产水平上进行检测和量化,这是努力减少人为排放的一个重大步骤性变化。Kayrros也向欧盟委员会及能源总局介绍了其方法和步骤。欧洲即将出台的减少甲烷排放战略将包括一种基于哥白尼计划和其他卫星数据的甲烷测量和量化方法,用于检测和验证。
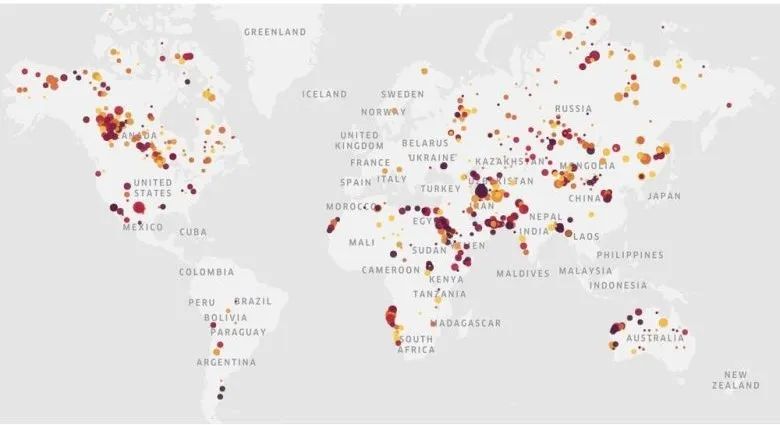
图2:Kayrros检测到的异常甲烷浓度样本,分析时间为2019年。圆圈的大小和颜色表示探测到的羽流的大小和强度。颜色越红,甲烷的浓度就越高。
(图片来源:Kayrros分析数据后进行的可视化图像,其中包含修改后的哥白尼数据)
这些研究的成果是巨大的,除了监测全球甲烷水平外,它们还使能源部门和个别公司能够识别并解决其网络中的甲烷泄漏问题。研究表明,世界上任何时候都有大约100个高排放量的甲烷泄漏。这些排放中约有50%来自石油和天然气、煤炭开采及其他重工业活动的地区。各国政府和监管机构应注意到,获得此类详细排放数据将大大提高它们追究排放者责任的能力,并有可能扭转排放率不断上升的趋势。例如Kayrros分析师在2020年1月发现了来自阿尔及利亚三个不同石油和天然气设施的烟羽。这些烟羽是由每小时排放超过25吨甲烷的气源引起的。如此大规模的甲烷泄漏大致相当于750兆瓦燃煤电厂的二氧化碳排放量。
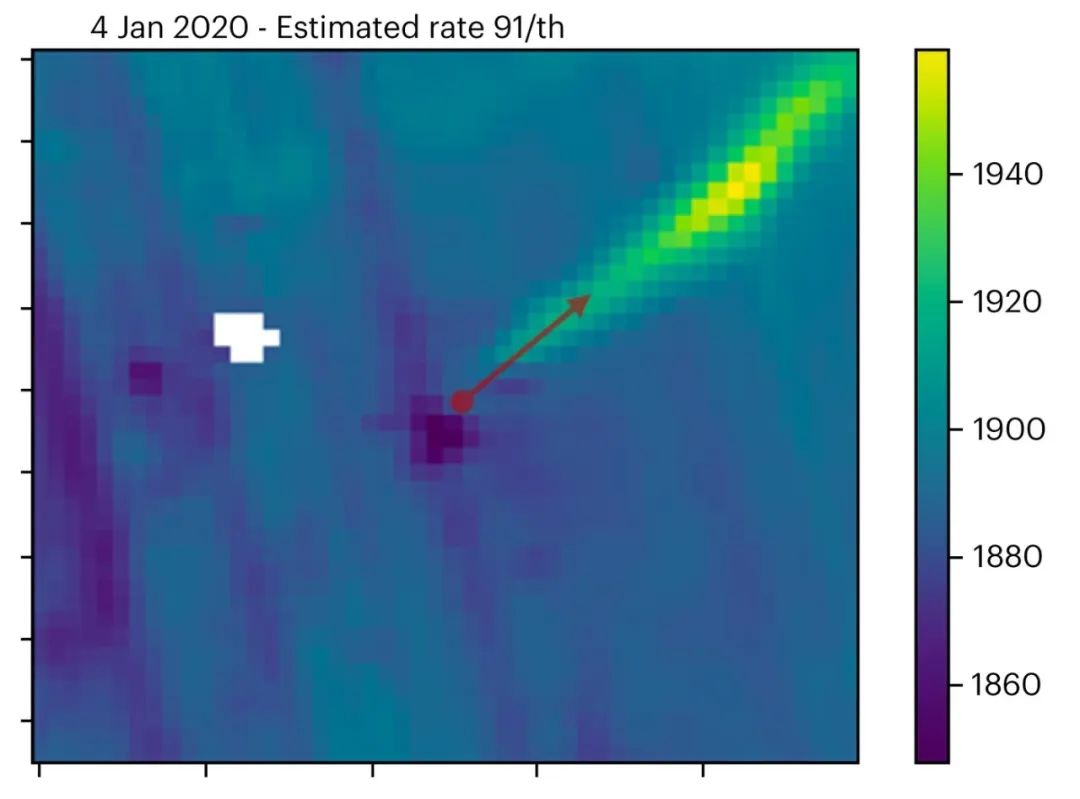
图3:2020年1月4日,Sentinel 5P在阿尔及利亚Hassi Messaoud附近观测到的甲烷羽流与Sonatrach的报道事件相吻合。红色矢量指示风向。
(图片来源:Kayrros,包含修改后的哥白尼数据)
卫星提供的数据和信息将继续提高全球研究人员对甲烷排放水平的认识。然而,卫星确实有一些局限性,例如现有卫星无法提供雪地、沼泽或近海地区的数据以及卫星通常只识别较大的排放源而无法捕获小规模的排放源等限制性问题。卫星识别的能力本身并不能解决甲烷排放所带来的挑战。解决这些问题需要公司维持减排标准,而决策者则要制定严格的法规。目前,行业和其他利益相关者都已经认识到,政策和法规可以在解决行动壁垒方面发挥关键作用。



内容转载、商务活动、投稿等合作请联系
邮箱:geomaticshtd@163.com
欢迎关注慧天地同名新浪微博:
ID:慧天地_geomaticser
《慧天地》敬告
以上是关于氮,氧,二氧化碳,天然气,氦,氖,氯 ,一氧化碳,二氧化硫的用处,出自那里,特性的主要内容,如果未能解决你的问题,请参考以下文章